Science in Translation Part I: What is Portable X-Ray Fluorescence Spectroscopy?
Helpful Abbreviation Key!
pXRF: Portable X-ray Fluorescent Spectroscopy
XRD: X-ray Powder Diffraction
PRS: Pecos River Style
Introduction
There are a lot of things about reading scientific journals, publications, and research reports that seem daunting. They might as well be in an entirely different language altogether as far as understanding goes, right? Don’t worry, you are not alone in this sentiment! That’s where I come in! I’m Bec, Shumla’s remote (thanks COVID-19. . .) intern for Dr. Karen Steelman for the Summer of 2020. I will be your personal translator and guide through our journey as we discuss a specific analysis method – portable X-ray fluorescence (pXRF) – that we use right in the field at different archaeological sites to study the rock art in the Lower Pecos region. No lab needed (BYO scientist, though)!
A quick little bit about me! My name is Bec Heyman, and I am a rising senior at Rhodes College in Memphis, Tennessee. Right now, I am working with Shumla from miles and miles away. I am a self-designed Studio Art, Art History, and Chemistry interdisciplinary major. All of the work I am doing now in these subjects will translate into my studies in graduate school, where I hope to complete a Masters of Science in Art Conservation! This means that I will be treating, restoring, fixing, and maintaining art pieces and historical artifacts, preserving them so that future generations may continue to experience the wonder of these works.
This job requires skills in the visual arts, knowledge of art history, and an understanding of chemistry; hence why I combined all of these studies into my interdisciplinary major. Shumla does so much work in all of these fields, so I hope that you can see how working with them is right up my alley. I am so grateful for this opportunity to learn and further my studies outside of the classroom!
In terms of Shumla’s archeological and art historical research, there are many ways scientists use analytical chemistry to find out important information about the materiality and composition of painted rock art (a.k.a. pictographs) and the surrounding landscape. In turn, these physical qualities shed light on the importance of material use in regard to classifying different styles of rock art, determining subject matter, identifying common iconography, and even piecing together the overall composition of these historical narratives. All of this information brings us a few steps closer to understanding the culture of the hunter-gatherers that occupied this area throughout the Holocene. So, buckle up. . . Our journey is about to begin!
Elemental Analysis
In the past, researchers have done studies and experimentation on Pecos River Style (PRS) rock art using a mineral analysis technique called X-ray diffraction (XRD). This analytical method requires a paint sample to be collected from the site and transported back to the laboratory for analysis and mineral identification. Using XRD, scientists concluded that most of the black pigment used for PRS rock art is comprised of various manganese minerals (manganite and pyrolusite). Whereas, red and yellow pigments are composed of primarily iron oxide or iron hydroxide minerals (hematite, goethite, etc.) (Hyman et al., 1996; Zolensky, 1982).
Elemental analysis means exactly what it sounds like: different methods that scientists use to determine what something is made of all the way down to its atomic level of materiality and identity – like the elements on the periodic table!
A recent study using an elemental analysis technique called pXRF has identified the presence of elements such as manganese and iron in over 248 paint samples at 10 rock art sites in the Lower Pecos Canyonlands (Koenig et al. 2014). This study confirmed the results from the XRD analysis but have, more importantly, allowed scientists to ask start asking different kinds of questions. Questions that go beyond “simple” elemental identification and extend into classifying, interpreting, and comprehending the rock art imagery in the region.
pXRF is very unique when compared to other analytical methods because it is one of the only systems that does not require destroying any part of the pictograph for the sake of sample analysis. This is crucial because most of the rock art in this region is constantly in danger of fading away forever due to environmental factors like flooding, rain, and mineral buildup on the paint surface.
Check out these blog posts to learn more about how Shumla uses chemical analysis to study the past:
Shumla’s Chemistry Lab Part I: Plasma Oxidation and Radiocarbon Dating
Shumla’s Chemistry Lab Part II: Radiocarbon Dating Eagle Cave Rock Art
Click the link below for more information about the deterioration of rock art in the Lower Pecos:
Time Does Not Heal All: Observable Deterioration in the Rock Art of Seminole Watering Hole (41VV72)
Breaking Down pXRF
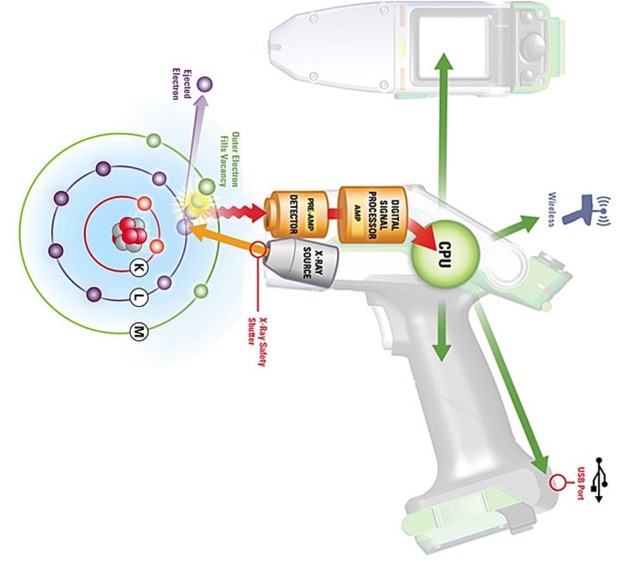
As the p in pXRF suggests, this instrument is portable! This feature is very rare when it comes to scientific tech and equipment. Most other elemental analysis devices are too bulky, complicated, etc. to take into the field, which, might I add, is always a very long hike away from the lab. It is super helpful being able to do analyses like this at the rock art site because the results are immediate.
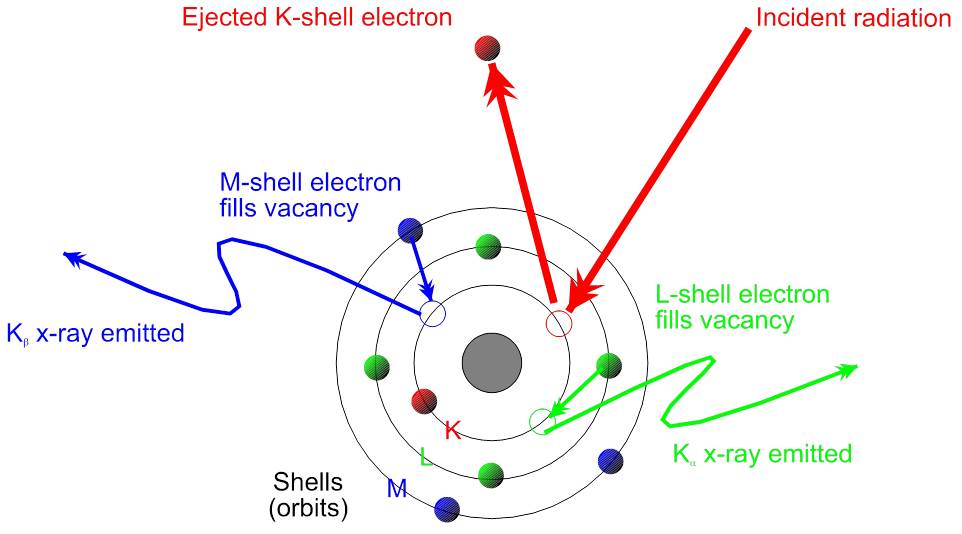
Moving along through our acronym, the X & r come together and stand for X-ray. Inside the device there is a special rod connected to the electricity source. The rod takes this electrical energy and converts it into primary X-rays (a.k.a. incident rays). These X-rays strike the sample, interacting with either paint or rock surfaces at an atomic level. X-rays are a form of energy, so when we are shooting energy at an atom, the little guy is just bound to get hyped up! First, these
incident rays kick out a low-energy electron found orbiting closest to the nucleus of the atom. The atom is now what we call “unstable.” But there’s no need to fear! Another electron, from the outer circle of electrons (also known as the outer orbital) that chill out far away from the nucleus, jumps in its former friend’s place to save the day and restore the atom’s stability! Our hero! As this electron uses its powers to jump closer to the nucleus, it releases its own kind of energy, which is a different form of X-rays called secondary rays.
This is where the next piece comes in: F is for fluorescence! The secondary X-rays I mentioned above are also called fluorescent rays. These are the rays released from our hero electron as it bounces to a lower orbital. Fluorescence is a big word that refers to the radiation – in our case the X-rays – that is emitted from the jumping electrons in the atoms of the sample as a result of being struck by the first set of radiation, the primary X-rays. Secondary X-rays are special because each ray is specific to a particular element. Think about it like its an autograph and each element has their own, unique signature. There is a detector in the pXRF device that can read these secondary X-ray signatures, and that is what tells us exactly which element is present in the sample.
That’s great and all, but acronym aside, what does spectroscopy mean? I, too, have wondered this, so I am so glad you “asked” . . . Spectroscopy refers to any analytical method that requires measuring the range of electromagnetic waves that are emitted from matter when that matter is exposed to electromagnetic radiation. pXRF is a type of spectroscopy because we are shooting out primary X-rays, making the surface interact with these radiation waves, and then reading the secondary X-rays that are emitted back off of that surface to determine elemental composition!
Science Does Not a Miracle Make
However, there is a slight “catch.” This kind of analysis only allows for the determination of the element(s) present in the test area. This method does not tell scientists anything about the molecular structure of the substance in question, simply that a certain element is present in the sample. This is important to know because it is extremely rare, if not impossible, to find a piece of pure iron just lying about in nature. For example, throughout Shumla’s research, scientists know every iron-based pigment used at these rock art sites are never just made of iron.
For example, in various pigments used on the pictographs in the Lower Pecos Canyonlands, the element iron (Fe) bonds with different elements in multiple ways, forming different minerals, each with its own unique structure producing a wide range of colors. The artists of these works would then use these minerals to create paint. Iron mineral derivatives include hematite (Fe2O3), limonite (FeO(OH) · nH2O), goethite (FeO(OH)), and magnetite (Fe3O4), if you wanted to know some specifics (Hymen et. al. 1996). The pXRF device tells us that iron (Fe) is one of the elements present in those paint samples, but it does not tell you which one of the minerals or a combination of those mentioned above is in this particular type of paint.
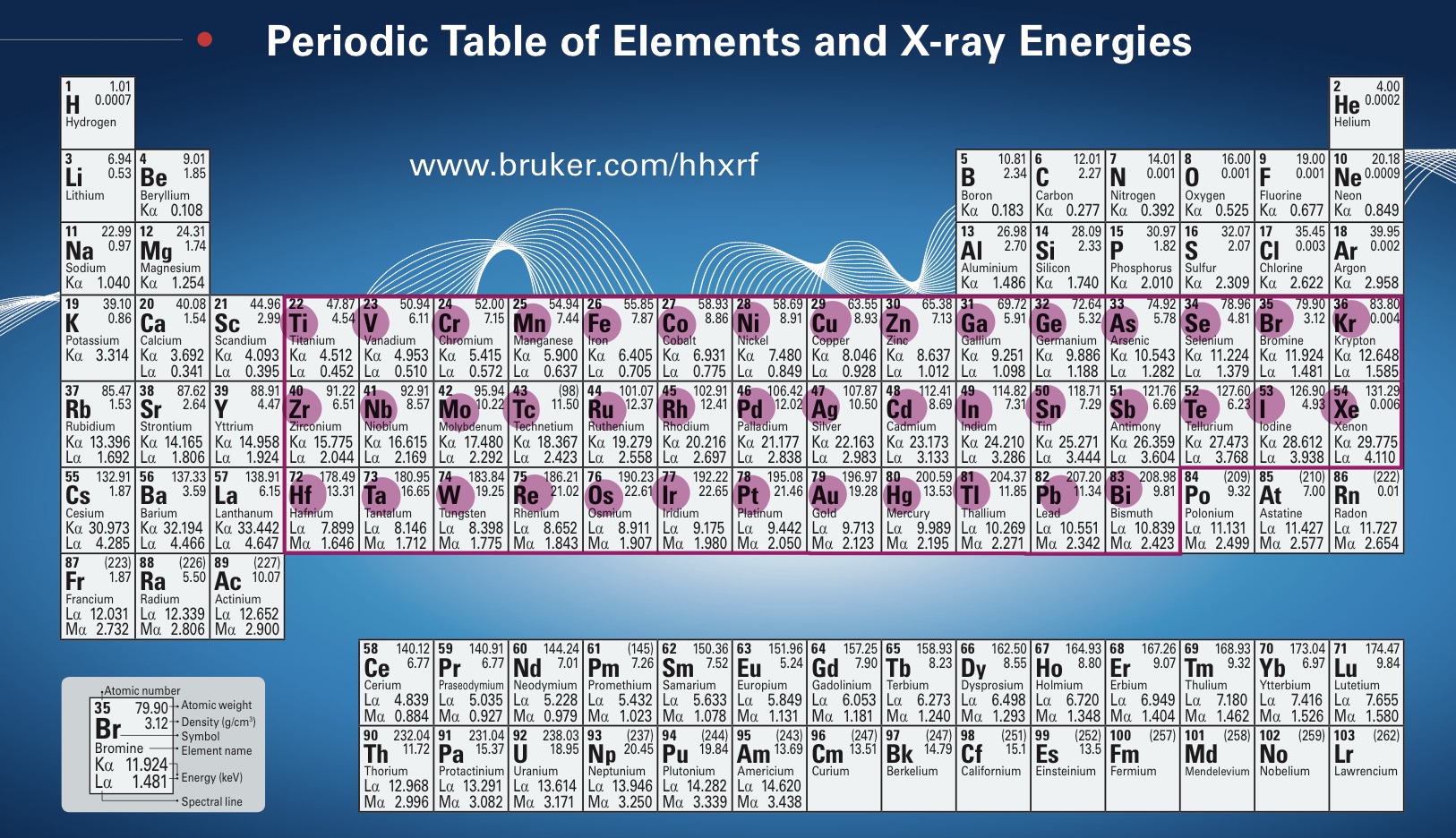
You may be wondering why we can identify the iron (Fe) in those minerals, but the device is not detecting the oxygen and hydrogen that also make up that mineral? Shouldn’t those elements also be detected, thus providing more specific information about these minerals and their structures? Sadly, science does not a miracle make. pXRF spectroscopy can only detect the elements titanium (Ti) through bismuth (Bi) on the periodic table. In other words, the pXRF device can only register elements from atomic numbers 22 through 83 (elements Ti through Bi).
Thanks for Staying Tuned!
I hope that this post helped everyone understand elemental analysis and pXRF a little better. The work that the scientists do in the lab at Shumla is truly amazing, and the world deserves to know about it. I will be continuing to post blogs throughout my internship with Shumla this summer. Stay safe everyone, and don’t forget to wear your masks!
Check out these publications from Shumla’s scientists for more comprehensive information on pXRF:
Portable X-Ray Fluorescence Analysis of Red Linear Style Figures at 41VV1000
Portable X-ray Fluorescence Analyses at the Meyers Spring Pictograph Site
References
Castañeda, Amanda M., Charles W. Koenig, Marvin W. Rowe, and Karen L. Steelman 2019, Portable X-Ray Fluorescence of Lower Pecos Painted Pebbles: New Insights Regarding Pigment Choice and Chronology. Journal of Archaeological Science Reports, 25:56-71.
Hyman, M., Turpin, S. A., and Zolensky, M. E., 1996, Pigment analyses at Panther Cave, Texas, Rock Art Research, 93-103.
Koenig, Charles W., Amanda M. Castañeda, Carolyn E. Boyd, Marvin W. Rowe, and Karen L. Steelman 2014 Portable X-Ray Fluorescence Spectroscopy of Pictographs: A Case Study from the Lower Pecos Canyonlands, Texas. Archaeometry, 168-186.
Zolensky, M., 1982, Analysis of pigments from prehistoric pictographs, Seminole Canyon State Historical Park, in Seminole Canyon: the art and the archaeology, 277–84, Texas Archeological Survey Research Report No. 83, The University of Texas Press, Austin, TX.






0 Comments