Shumla’s Chemistry Lab Part I: Plasma Oxidation & Radiocarbon Dating
**This post is the first of two blog posts describing the Shumla Chemistry Lab and our plasma oxidation system.**
By Karen L. Steelman
In my role as Shumla Research Director, I have been working alongside the team on The Alexandria Project, as well as pursuing research that is near and dear to my heart – radiocarbon dating of rock art.
Radiocarbon Dating Basics
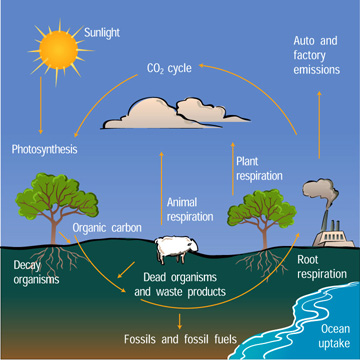
Radiocarbon dating is a method scientists use to calculate the age of a once living material by measuring the amount of the radioactive carbon isotope, C-14, in relation to the stable carbon isotopes of C-13 and C-12. Radiocarbon (C-14) is created via cosmic rays in the Earth’s upper atmosphere, and is much less common than C-13 or C-12. All three carbon isotopes are incorporated into living organisms throughout their lives as part of the carbon cycle. Once an organism dies, the C-14 isotopes decay at a known steady rate, called a half-life. Radiocarbon has a measured half-life of about 5730 years, which means that every 5730 years one-half of the C-14 decays via a β– emission.
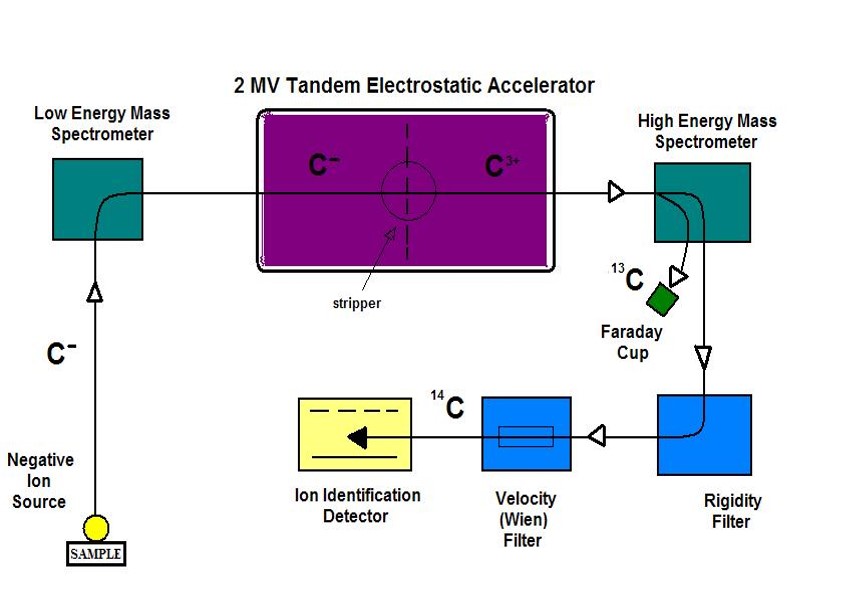
Originally, radiocarbon dating was done by measuring that β– emission. However, a more sensitive technique is now available to analyze smaller samples by directly measuring the ratio of C-14 that remains in a sample and the stable isotopes of carbon, using a technique called accelerator mass spectrometry. This ratio of C-14/C-13 is related to a sample’s age. Then, scientists are able to determine the age of a sample, providing a radiocarbon assay, or radiocarbon date, for the organic sample. To learn more, check out the webpages from Beta Analytic and radiocarbon web-info as well as the video, Carbon Dating Explained.
Why is Dating Rock Paintings Different?
Unlike with “dirt” archaeology where the preservation of organic materials is relatively common, pictographs present a distinct challenge for radiocarbon dating because there is very little organic material. Most paints are comprised of three primary ingredients: pigment (color), binder (holds the pigment together), and an emulsifier (thins the binder/pigment mixture into a paint). Therefore, any radiocarbon assay on rock paintings targets the organic components of the paint. However, this is a challenge because most rock art assemblages around the world contain mineral pigments rather than organic pigments. For instance, the black color of Lower Pecos rock paintings are from manganese minerals, while red and yellow pigments are made with iron minerals. The challenge for chemists was to develop a technique to isolate the organic components from the inorganic constituents within rock paintings to allow a radiocarbon assay on only the organic components.
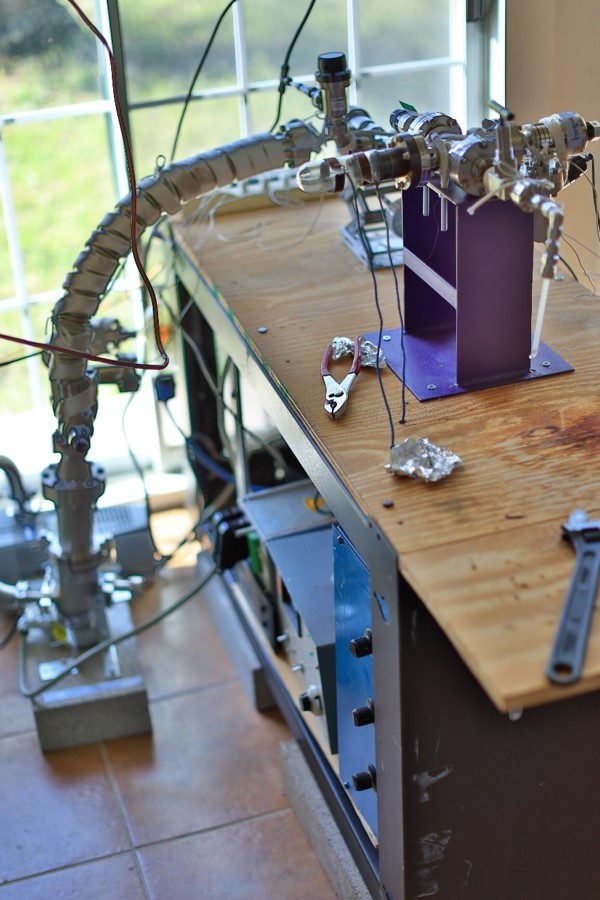
Plasma Oxidation and the Shumla Chemistry Laboratory
In the 1990s my former graduate advisor, Dr. Marvin Rowe, and his chemistry graduate students at Texas A&M University developed and pioneered the plasma oxidation technique to directly date pictographic rock art. Using samples collected mainly from 41VV75 in Seminole Canyon State Park and Historic Site, Marvin and his graduate students utilized the plasma oxidation method to extract the organic binders and emulsifiers in the paint. These samples from 41VV75 became some of the first directly dated pictographs anywhere in the world!
When I took the position as Research Director, I knew that I would be establishing a Shumla Chemistry Laboratory. The first instrument I built was a plasma oxidation system so Shumla could begin preparing paint samples for radiocarbon dating. I’ve made some changes to Marvin’s original design, but the basic principal remains the same: employ plasma oxidation of the organic binders/emulsifiers in the paint layer to extract organic material for radiocarbon dating.
How Does Plasma Oxidation Work?
Step 1:
Place sample into glass chamber
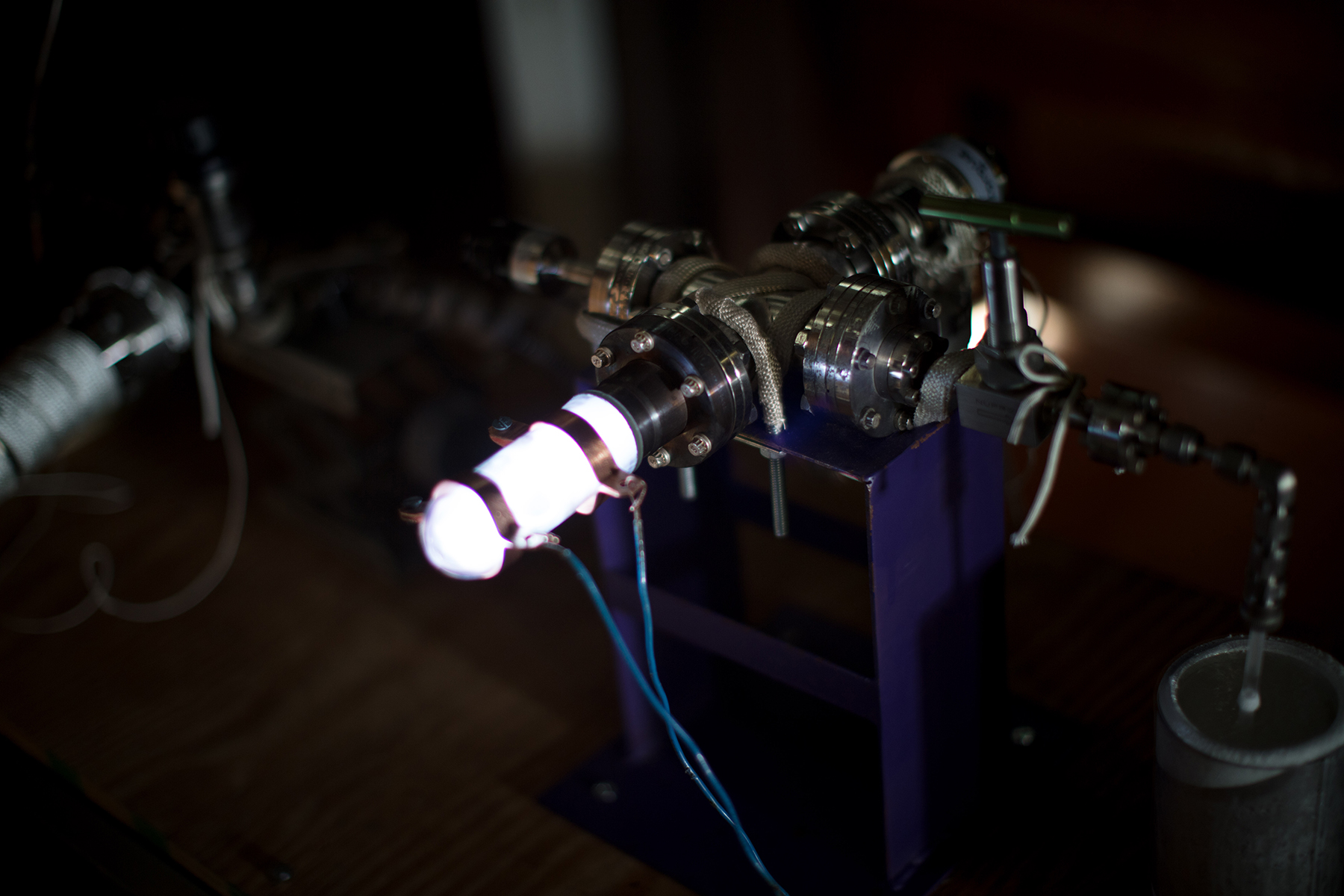
Steps 2 and 3:
Fill chamber with argon or oxygen (depending on the process). Apply electricity to the gas inside the chamber. It is called a low-temperature plasma because it is an ionized gas…much like a neon sign that glows.
Step 4:
Electrically excited oxygen is used to oxidize or convert organic material in the paint sample to carbon dioxide (CO2) and water.
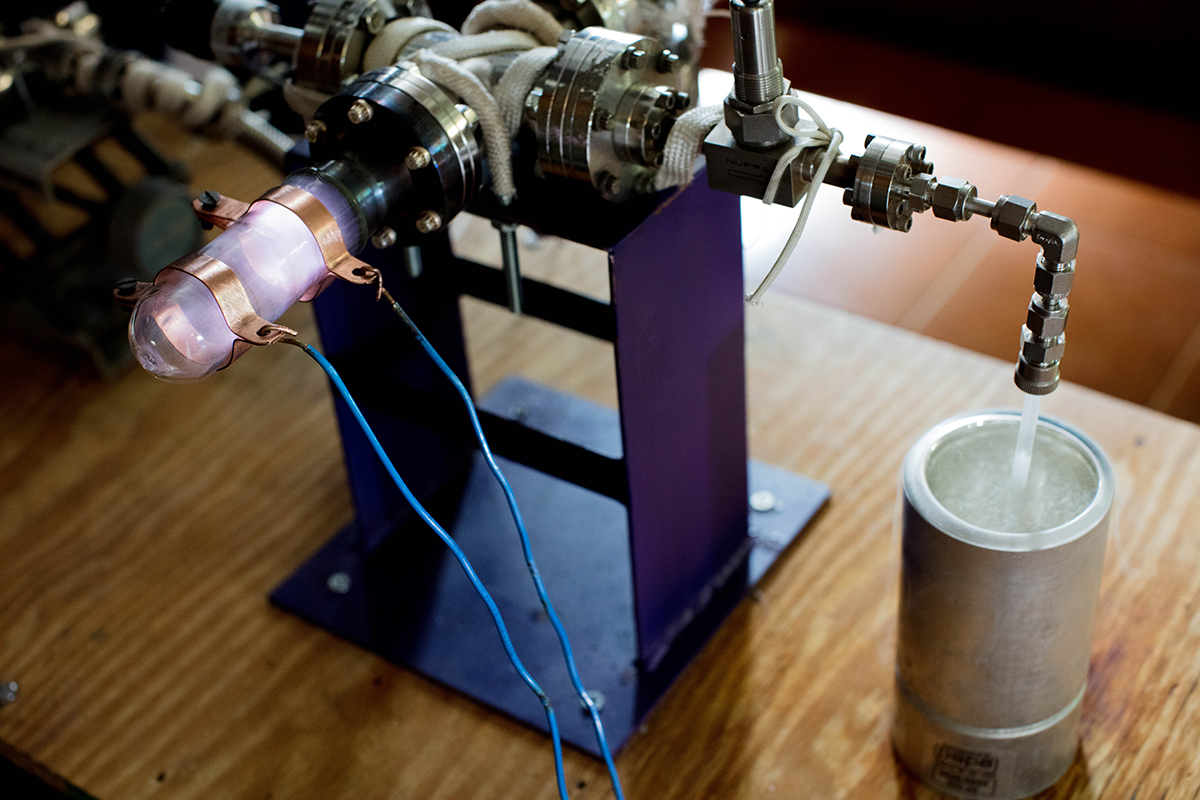
Step 5:
The glass tube is immersed in liquid nitrogen in order to freeze the gas CO2 to dry ice.
Step 6:
Seal off the tube of CO2 using a blow torch.
Step 7:
Send glass tube of CO2 off for radiocarbon dating at an Accelerator Mass Spectrometry (AMS) laboratory.
The Plasma Oxidation Advantage
One beneficial aspect of plasma oxidation over other sample preparation techniques is that only the organic carbon is extracted, leaving intact the inorganic portion of the paint sample because the system remains below the decomposition temperature of these minerals. This allows us to avoid the extensive acid treatments that traditional sample preparation and combustion use. And, we are able to analyze much smaller samples with high mineral content, which is ideal for dating paint that is still adhering to rock! Once the carbon dioxide is extracted, it is sent for radiocarbon dating using AMS. I have collaborated with Lawrence Livermore National Laboratory’s Center for Accelerator Mass Spectrometry (CAMS) for over 20 years.
Ongoing Shumla Radiocarbon Research
Since the 1990s, the focus of rock art dating in the Lower Pecos has been on dating Pecos River Style paintings, with 33 radiocarbon assays from 9 different sites. The radiocarbon dates for Pecos River Style range from approximately 2700 B.C. to A.D. 600 (Bates et al. 2015). With this data set, the Lower Pecos Canyonlands is considered one of the best dated rock art provinces in the world. However, most of these samples were collected in the 1990s, and in many ways this early work was experimental — Marvin and his students were demonstrating that plasma oxidation could be utilized to directly date rock paintings. But, because the Lower Pecos Canyonlands was the first place that plasma oxidation was used to radiocarbon date rock paintings, and the focus was on developing the technique, there was very little focus on answering archaeological research questions. For most of the dated samples, we know nothing more about the sample than the radiocarbon date itself. In other words, we have no information on the rock figures which were dated. This is a crucial piece of missing information because one of the research projects I and other Shumla researchers are most interested in pursuing is determining chronological distribution of specific rock art images and motifs across the Lower Pecos landscape.
The Shumla Chemistry Lab Part 2: Plasma Oxidation and Eagle Cave Pictographs
Stay tuned for Part 2 of the Shumla Chemistry Lab blog which will detail how we used plasma oxidation to date the rock art at Eagle Cave, and how we plan to use radiocarbon dating alongside The Alexandria Project to enhance our knowledge of Lower Pecos chronology.












Fascinating read. Looking forward to part tweux.
Will this method of datation be used eventually on sites around the world? European cave art?
Hi Didier! Yes, plasma oxidation has been used to extract carbon from pictographs at many sites across the world.